- Home
- Product
- Seawater desalination equipment
- EDI ultrapure water equipment
- Laboratory ultrapure water machine
- Ultrafiltration equipment
- Sewage treatment equipment
- Container integrated water purification equipment
- Double-stage reverse osmosis equipment
- Pure water equipment
- Single-stage reverse osmosis equipment
- Medical purified water equipment
- Hospital pure water equipment
- Ultrapure water equipment
- Recycled water reuse equipment
- Commercial pure water machine
- About
- Case
- News
- Contact
Over the past 5 years, we have solved water problems for tens of thousands of enterprises
产品展示
products
- Sewage treatment equipment
- Laboratory ultrapure water machine
- Ultrapure water equipment
- EDI ultrapure water equipment
- Medical purified water equipment
- Hospital pure water equipment
- Pure water equipment
- Single-stage reverse osmosis equipment
- Double-stage reverse osmosis equipment
- Seawater desalination equipment
- Ultrafiltration equipment
- Container integrated water purification equipment
- Recycled water reuse equipment
- Commercial pure water machine

2T purified water equipment, dual-stage RO purified water system, EDI industrial medical cosmetics industry purified water machine
Equipment for preparing pure water to meet medical needs. The entire system is also made of all stainless steel, and sterilization devices must be equipped before the water point. Using reverse osmosis, EDI and other processes, a complete set of high-purity water treatment processes are designed in a targeted manner to meet the water requirements of purified water production and large infusion production in pharmaceutical factories and hospitals.
Equipment for preparing pure water to meet medical needs. The entire system is also made of all stainless steel, and sterilization devices must be equipped before the water point. Using reverse osmosis, EDI and other processes, a complete set of high-purity water treatment processes are designed in a targeted manner to meet the water requirements of purified water production and large infusion production in pharmaceutical factories and hospitals.

GMP Purification Water Equipment Introduction
Equipment for preparing pure water to meet medical needs. The entire system is also made of all stainless steel, and sterilization devices must be equipped before the water point. Using reverse osmosis, EDI and other processes, a complete set of high-purity water treatment processes are designed in a targeted manner to meet the water requirements of purified water production and large infusion production in pharmaceutical factories and hospitals.
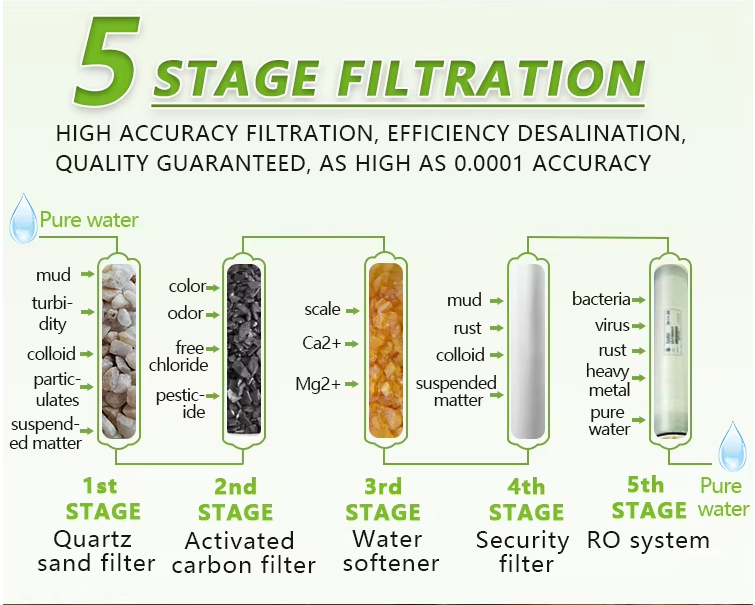
Purified water equipment process flow

Raw water → raw water tank → raw water pressurization pump - multi-media filter → activated carbon filter → cationic water softener → precision filter → first-stage booster pump → first-stage reverse osmosis → intermediate water tank → second-stage booster pump → second-stage reverse osmosis − purified water storage tank → purified water pressurization pump → ultraviolet sterilizer → microporous filter → water point.
Purification water equipment parameters
Water inlet requirements | Municipal tap water |
Water quality | The effluent water quality : conductivity (25°C water temperature) <5.1us/cm, total organic carbon <0.5mg/L |
Water production | 2000L/H, (25℃ water temperature) |
Recovery rate | 60-65% |
Raw water inlet water pressure | 0.2-0.4MPa |
Applicable water temperature | 10-40℃ |
power | 4.5KW |
Suitable power supply | AC380V 50HZ |
External dimensions | 1400*800m*1500(Length*Width*H) |
Interface size | Water inlet: 32 Wastewater outlet: 25 Water purification outlet: 25 |
Conditions to meet the purification equipment
1. The structural design of GMP purified water equipment should be simple, reliable, and easy to disassemble and assemble.
2. To facilitate disassembly, assembly, replacement and cleaning of parts, the design of the actuator should adopt standardized, generalized and systematic parts as much as possible.
3. The inner and outer wall surfaces of the equipment must be smooth and smooth, without dead corners, and are easy to clean and sterilize. The surface of the parts should be treated with chrome or other surface treatment to resist corrosion and prevent rust. Avoid paint on the outside of the equipment to prevent peeling.
4. GMP purified water equipment should use low-carbon stainless steel or other materials that are proven to not contaminate water quality. GMP purified water equipment should be cleaned regularly and the cleaning effect should be verified.
5. The material contacting water for injection must be high-quality low-carbon stainless steel (such as 316L stainless steel) or other materials that have been proven to not contaminate water quality. Equipment for preparing injection water should be cleaned regularly and the cleaning effect should be verified.
6. The storage period of purified water produced by GMP purified water equipment should not be greater than 24 hours. The storage tank should be made of stainless steel or other materials that are proven to be non-toxic, corrosion-resistant, and do not exudate polluted ions. A hydrophobic sterilization filter that protects its ventilation port should be installed without falling off the fibers. The inner wall of the storage Yao should be smooth, and there should be no dead corners or trachoma on the pipes and welds. Sensors that will not cause stagnant water pollution to show liquid level, temperature pressure and other parameters should be used. Chuyao should be cleaned, disinfected and sterilized regularly, and the effects of bay washing and sterilization should be verified.
Reverse osmosis equipment process flow chart






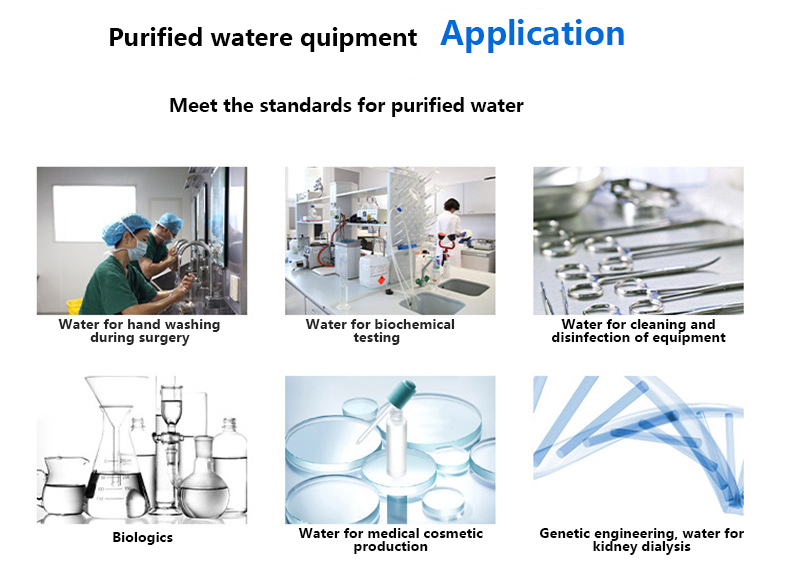
what app
Address: No. 9655, Tingwei Highway, Jinshan Tinglin Industrial Zone, Shanghai
Mobile phone: +86 17717591248
WhatsApp: +852 5534 9894
Email: bosen7298@gmail.com
- Home
- Product
- Seawater desalination equipment
- EDI ultrapure water equipment
- Laboratory ultrapure water machine
- Ultrafiltration equipment
- Sewage treatment equipment
- Container integrated water purification equipment
- Double-stage reverse osmosis equipment
- Pure water equipment
- Single-stage reverse osmosis equipment
- Medical purified water equipment
- Hospital pure water equipment
- Ultrapure water equipment
- Recycled water reuse equipment
- Commercial pure water machine
- About
- Case
- News
- Contact



